
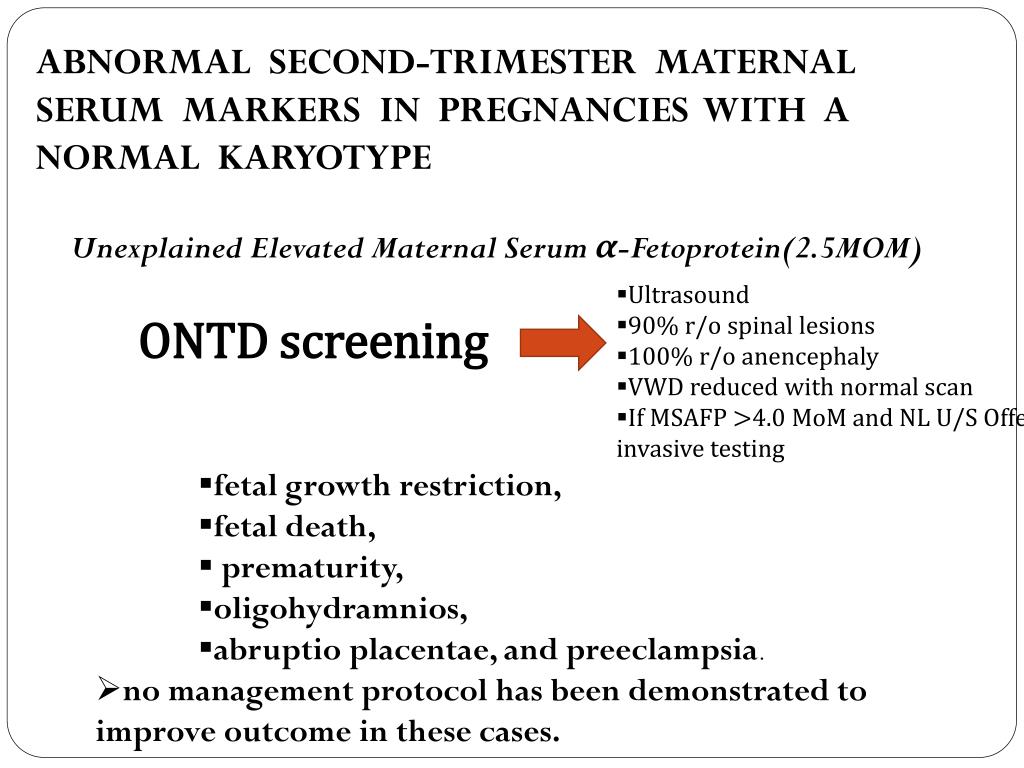
- #Sequential screening with nuchal fold translucency plus#
- #Sequential screening with nuchal fold translucency free#
Maternal fetal-derived circular RNA (circRNAs).First-trimester maternal serum placental growth factor level.
First-trimester maternal serum anti-Mullerian hormone level.First-trimester maternal serum A disintegrin and metalloprotease 12 (ADAM12-S) level.First-trimester maternal plasma levels of follistatin-related gene protein.First-trimester ultrasound assessment of the nasal bone.First-trimester serum analyte testing (hCG Footnote* and PAPP-A) alone without NT measurement.First-trimester NT measurement alone (without first-trimester serum analyte testing) in the absence of fetal cystic hygroma in singleton pregnancies.
#Sequential screening with nuchal fold translucency free#
See CPB 0464 - Serum and Urine Marker Screening for Fetal Aneuploidy and CPB 0787 Comparative Genomic Hybridization (CGH).įootnotes1* For purposes of this policy, these various forms of hCG are considered interchangeable: free beta subunit of hCG, total hCG, or hyperglycosylated hCG (also known as invasive trophoblast antigen ).Īetna considers other non-invasive screening schemes for fetal aneuploidy to be experimental and investigational, including the following because their effectiveness has not been established:
Measurement of cell-free fetal nucleic acids in maternal blood when criteria are met in CPB 0464 - Serum and Urine Marker Screening for Fetal Aneuploidy.Īetna considers the above-listed screening tests for fetal aneuploidy not medically necessary for women who have previously had a microarray or non-invasive prenatal testing (NIPT) with cell-free DNA during the current pregnancy. #Sequential screening with nuchal fold translucency plus#
Serum integrated screening for pregnancies where NT measurement is not available or can not be obtained: First-trimester (PAPP-A plus hCG Footnote*) plus second-trimester quad (MSAFP, uncongugated estriol, inhibin A, and hCG Footnote*) screening or. Second-trimester serum analyte screening (see CPB 0464 - Serum and Urine Marker Screening for Fetal Aneuploidy) or. Integrated, sequential, or contingent screening: First-trimester triple test (NT, PAPP-A, and hCG Footnote*) plus second-trimester quadruple test (maternal serum alfa-fetoprotein (MSAFP, unconjugated estriol, inhibin A, and hCG Footnote*) screening or. First-trimester NT measurements results combined with the results of first trimester serum analyte tests that include pregnancy-associated plasma protein A (PAPP-A) plus beta-human chorionic gonadotropin (hCG) Footnote* or. First-trimester nuchal translucency (NT) testing alone (without serum analyte screening) for multiple gestations or. This Clinical Policy Bulletin addresses noninvasive down syndrome screening.Īetna considers the following noninvasive screening schemes for fetal aneuploidy medically necessary: 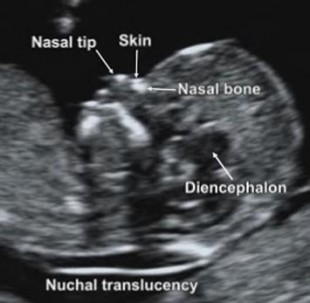
Number: 0282 Table Of Contents Policy Applicable CPT / HCPCS / ICD-10 Codes Background References







 0 kommentar(er)
0 kommentar(er)
